
Therefore the electronegativity is greatest at the top-right of the periodic table and decreases toward the bottom-left. The number of neutrons for the Bohr diagram of Oxygen can be found by subtracting the number of protons from the atomic mass (rounded to the nearest whole). Now the electron configuration of oxygen shows that the last orbital of oxygen has four electrons (2p 4 ). Oxygen is neutral and its atomic number is 8, hence, the number of protons and electrons available for its Bohr diagram is also 8. Elements with high ionization energies have high electronegativities due to the strong pull exerted by the positive nucleus on the negative electrons. That is, the atom of the oxygen element has a total of eight electrons. Electrons with low ionization energies have low electronegativities because their nuclei do not exert a strong attractive force on electrons. Electronegativity is related with ionization energy and electron affinity. The most electronegative atom, fluorine, is assigned a value of 4.0, and values range down to cesium and francium which are the least electronegative at 0.7. If it is stable it should exist on the earth.
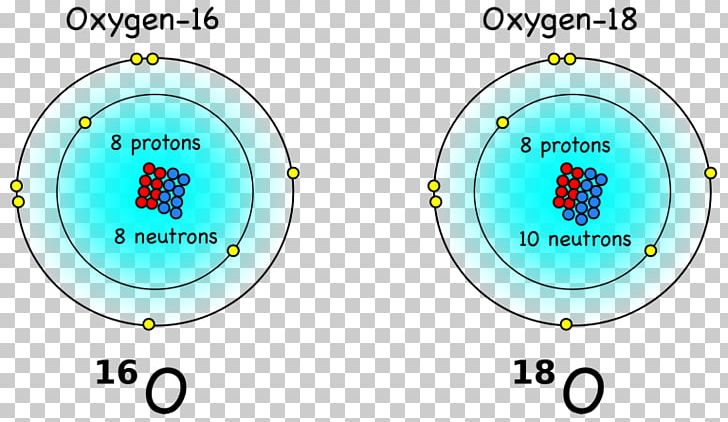
It ought therefore to be an isotope of oxygen. The higher the associated electronegativity number, the more an element or compound attracts electrons towards it. It must however have a mass 17, and provided no other nuclear electrons are gained or lost in the process, an atomic number 8. In general, an atom’s electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The electronegativity of Oxygen is: χ = 3.44 For this purposes, a dimensionless quantity the Pauling scale, symbol χ, is the most commonly used. to lose electrons for the reaction with chlorine or oxygen Lithium has a electron configuration of 2,1 and potassium 2,8,8,1 ( higher atomic number). You will need to refer to a periodic table for proton values.Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom.

In this notation, the atomic number is not included. Complete answer: The atomic number or proton number of any chemical element is the.

Symbol-mass format for the above atom would be written as Cr-52.
For an example of this notation, look to the chromium atom shown below:Īnother way to refer to a specific atom is to write the mass number of the atom after the name, separated by a hyphen. The "A" value is written as a superscript while the "Z" value is written as a subscript. Both the atomic number and mass are written to the left of the chemical symbol. In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. It is a member of the chalcogen group on the periodic table and is a highly.
Oxygen atomic number free#
Thus, we use O2 as the formula for oxygen, N2 for nitrogen, and so on. Oxygen is the eighth element with a total of 8 electrons. Because neutral atoms in the periodic table have the same number of electrons as protons, the atom must have 8 protons. Free Essay: Oxygen is a chemical element with symbol O and atomic number 8. The composition of any atom can be illustrated with a shorthand notation called A/Z format. Those nonmetals that occur as diatomic (two-atom)molecules are listed in Table 3.6.


 0 kommentar(er)
0 kommentar(er)
